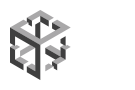3i platforms and technologies come together to enable the latest fluorescence microscopy methods and techniques.
OPTOGENETICS
Opto-mechanical devices for holographic photostimulation via spatial light modulation and high speed diffraction limited point scanning, both integral to VIVO Multiphoton™ and Marianas™ imaging platforms.
RATIOMETRIC IMAGING
Ratio imaging of FURA and other ratio dyes.
Optogenetics
Opto-mechanical devices for holographic photostimulation via spatial light modulation and high speed diffraction limited point scanning, both integral to VIVO Multiphoton and Marianas imaging platforms.
Phasor
Phasor is the only commercially available system capable of simultaneously illuminating multiple arbitrary regions in 3D with the use of SLM-based computer-generated holography (CGH).
Phasor adds precise and powerful photostimulation with advanced optical correction capabilities for maximum control of region shape, intensity, and location in 3D.
Phasor with LaserStack enables reliable, rapid 1P stimulation with up to 7 wavelengths. Visible wavelengths can be combined with near-IR for 2P uncaging at 720 nm.
Phasor 2P provides near-IR stimulation for deeper penetration in scattering tissue and 2-photon optical sectioning limits out-of-focus excitation. Zero-order management and diffraction correction produce an even power distribution with no dark spot. Temporal Focusing for 2P confines the axial illumination to a thickness less than 20µm, resulting in a consistent z resolution independent of region size.
Temporal Focusing for 2P confines the axial illumination to a thickness less than 20µm, resulting in a consistent z resolution independent of region size.
Screen capture of the preBotC network with neurons of interest selected for simultaneous photostimulation via Phasor to elicit a network burst.
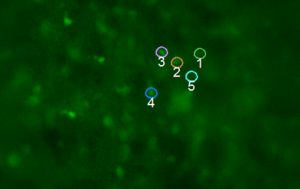
Ephys recording where the bottom trace represents the laser pulse and the top trace is a recording of the hypoglossal nerve (the output of the preBotC network). Arrows are pointing to endogenous bursts, while evoked bursts are indicated by the timing of the laser pulse.
Image and data courtesy Dr. Jason Worrell, UCLA
Vector
Vector executing an automatically generated sequence of stimulation events in a grid pattern for brain circuit mapping using glutamate uncaging or ChR2 activation. Order is generated to maximize spatial separation between any two subsequent stimulation locations.
Vector is a high speed galvanometer-based point scanner capable of delivering powerful and finely controlled laser light to user-defined areas in a specimen. Coupled with LaserStack, it can deliver a diffraction limited spot of intense laser light to a prescribed spot, region or multiple regions from a variety of laser wavelengths across the visible spectrum.
Vector is highly effective for uncaging and photobleaching. Coupled with Ablate!™, it can provide powerful pulsed laser ablation and laser-induced injury. Vector is also central to 3i’s VIVO Multiphoton imaging system.
SlideBook’s control of Vector and Phasor allows synchronization of photostimulation with imaging and electrophysiology with microsecond-level timing precision. SlideBook 5.5 introduces numerous enhancements:
• Direct recording of electrophysiological data for perfect correlation with imaging
• Ultra-precise, flexible control of optogenetic and caged compound stimulation
• Automatic grid pattern sequencing for neural circuit analysis
• Multichannel streaming capture
• Extended interoperability with MATLAB®
VIVO Multiphoton
VIVO Multiphoton is designed for high-speed, high-sensitivity multiphoton imaging of live animals and tissue. Incorporating the Vector scanning system, it can acquire whole fields, sets of arbitrary regions, curves or lines, all with synchronized piezo Z focus and power modulation. VIVO Multiphoton can collect multiple volumes per second, with laser power optimized for varying depths and eliminating any excitation in unwanted regions.
VIVO Multiphoton offers many detection options, including a proprietary nosepiece that places two GaAsP PMTs close to the objective and a substage unit that places two GaAsP PMTs close to a high NA water condenser automatically tracked to the nosepiece position. There are many ways to integrate photostimulation, using either 3i’s LaserStack or a second multiphoton laser, which can be addressed by the same Vector, a second Vector, or Phasor.
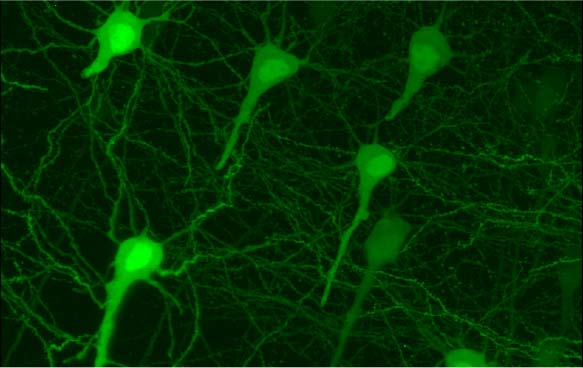
GFP transfected hippocampal neurons acquired by students at the 2012 CSHL course Imaging Structure and Function of the Nervous System
Key differences between a digital micromirror device (DMD), Vector laser-based ROI scanner, and the Phasor digital holography system:
| Masking With DMD | Scanning (3i Vector) | Holography (3i Phasor) | |
|---|---|---|---|
| Photomanipulation Methods | |||
| Optogenic Photostimulation | |||
| Uncaging | |||
| Photobleaching (FRAP) | |||
| Laser Ablation | |||
| Laser Induced Injury | |||
| Photoconversion | |||
| Photoactivation | |||
| Photoswitching | |||
| Multiphoton | |||
| 3D Illumination | |||
| Simultaneous Multipoint Illumination |
Excellent
Good
Limited
Not Applicable
| Performance Specifications | |||
|---|---|---|---|
| Power at single point | 1-2 µW | 10-20 mW | 10-20 mW |
| Fastest 1/4 field illumination | < 1ms | 100 ms | 1 ms |
| Multiple Wavelength Illumination | 1-2 Colors | 6+ Colors | 6+ Colors |
Fluorescence-lifetime
imaging microscopy
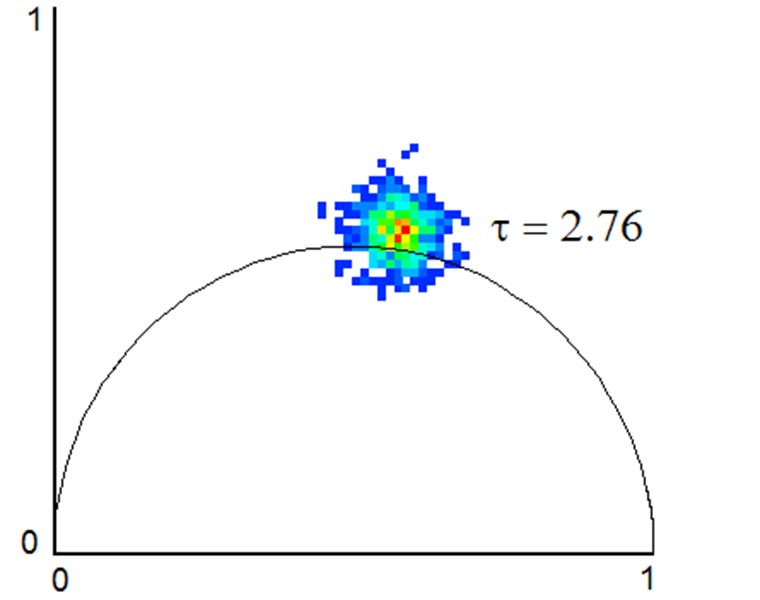
FLIM enables measurement of fluorescence lifetimes via frequency modulation of illumination and detection signals. FLIM is a powerful tool for molecular research in living cells and can be used to measure protein proximity (FRET), polymerization, relative concentration of different molecules, separation of different labels with spectral overlap, ion concentration, and remove autofluorescence. Lifetimes are measured at the sub-nanosecond level by using unique electronic timing circuitry for synchronized phase-shifted illumination. Near single molecule detection is possible.
The 3i FLIM system can be used in combination with other multidimensional imaging techniques and images orders of magnitude faster than time domain systems. This system is based on widefield frequency-domain detection, which uses a high frequency modulated laser light source and intensifier gated CCD detector. It is fully integrated and automated, combinable with widefield fluorescence, TIRF, FRAP, spinning disk confocal and multiphoton imaging./p>
A lifetime image can be acquired in less than a second, making it several orders of magnitude faster than a point scanning photon counting system. This speed is particularly useful for monitoring lifetime dynamics in living cells and tissue. FLIM analysis is closely integrated into 3i’s SlideBook™ software. Through an intuitive polar plot (vector) graphical analysis, it is easy to visualize multiple fluorescent species, FRET efficiency, and mixture analysis.
The FLIM system is available as a module of our Marianas microscope workstations. It can be combined with all capture types (e.g. time lapse, 3D, multiple locations) and different microscopy modalities such as widefield, deconvolution, spinning disk confocal, multiphoton and TIRF.
Förster Resonance
Energy Transfer
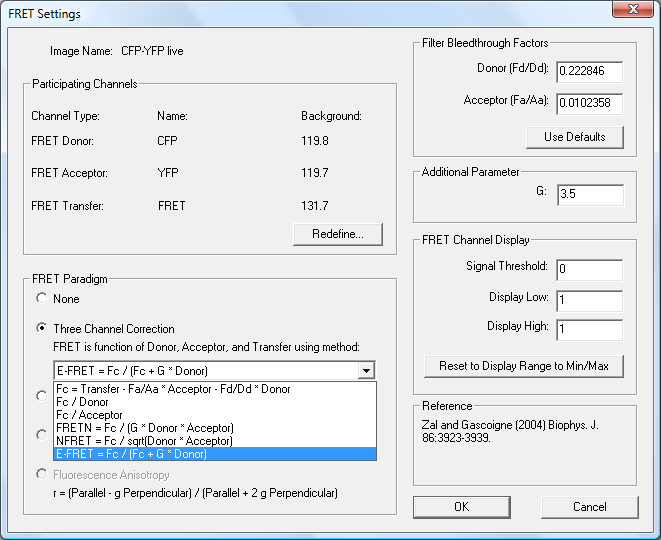
SlideBook’s FRET module contains analysis tools for sensitized emission FRET and acceptor photobleaching FRET. Real-time FRET analysis is possible with simultaneous dual channel capture via image splitting devices or dual camera setups.
Graphs are displayed and updated during capture. Features include channel bleed through calculation, background subtraction, corrected FRET image generation (Herman equation), single pixel to whole object energy transfer measurement, and support for 2D, 3D and 4D data. Fluorescence anisotropy measurement is also possible and fluorescence lifetime FRET is available via the FLIM Module.
Ratiometric imaging
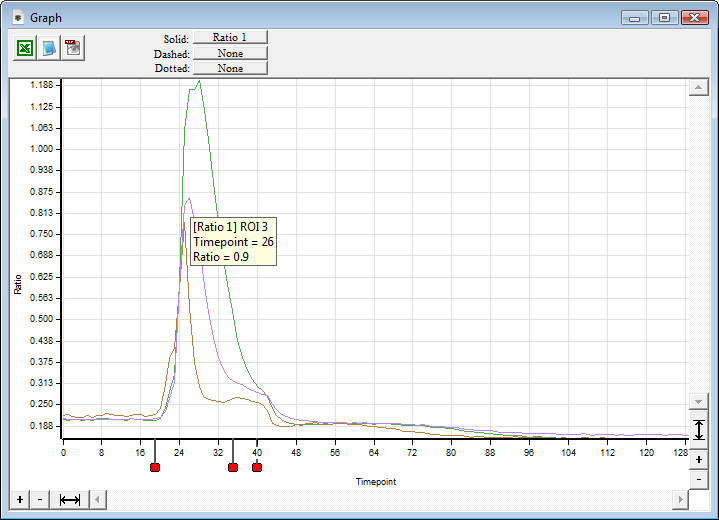
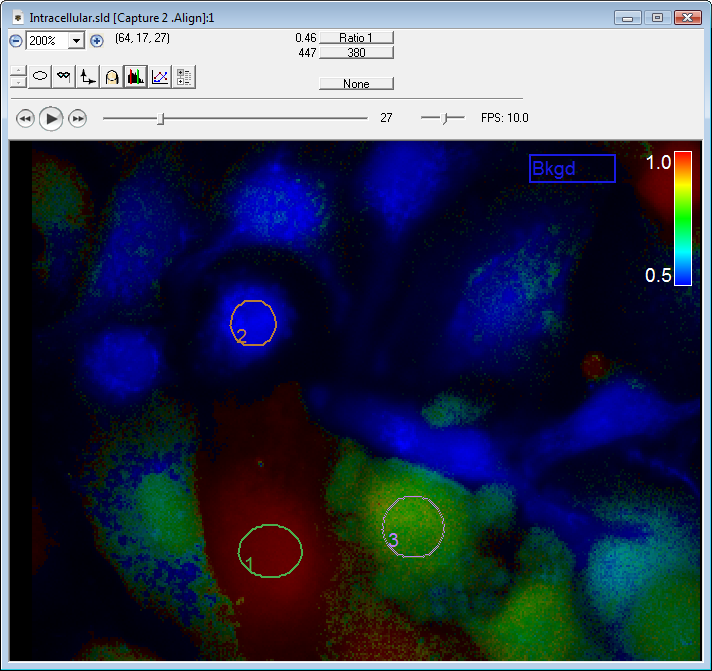
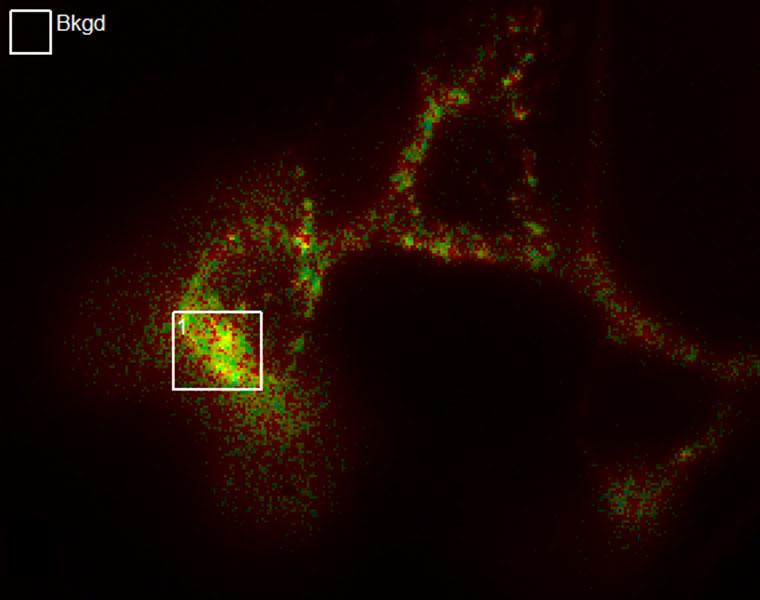
SlideBook’s Ratio Imaging module allows calibration, acquisition, graphing, and analysis of data from ratiometric indicators such as Fura-2, BCECF, and genetically expressed Cameleons. Ratio data can be uncalibrated, calibrated using buffer solutions, or calibrated intracellularly. Ratio display and statistics are performed live using floating point arithmetic on the raw image data to minimize roundoff error and utilize memory efficiently. Background region can be adjusted after acquisition if spurious signal enters the originally selected background region.
SlideBook allows ratio channels to be collected alongside other fluorescence imaging channels allowing elaborate 3D and 4D experiments incorporating ratio information. It is possible to collect 4D data in GFP and select one plane of each stack to collect Fura-2 data. Ratio imaging can also be part of elaborate experiments which combine ratio acquisition with non-ratiometric labels, 3D and 4D imaging and synchronization with electrophysiology and automated perfusion.