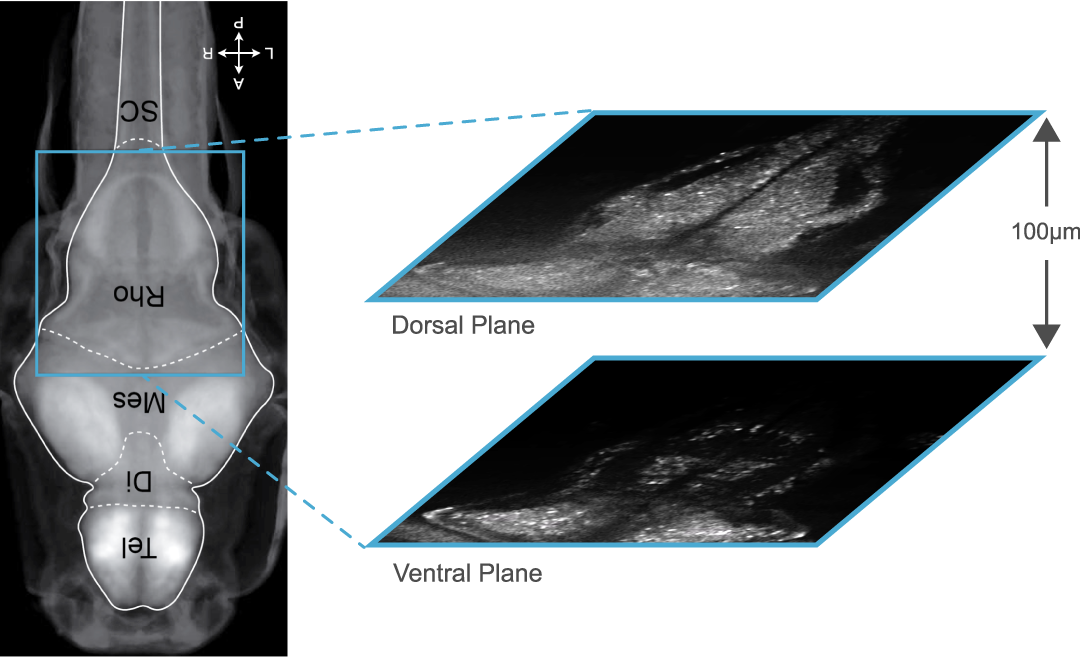
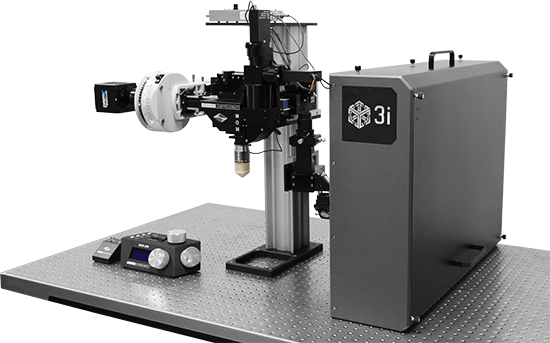
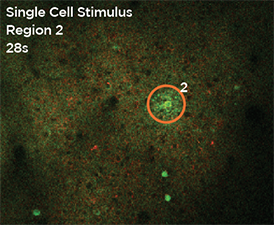
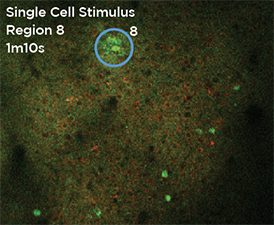
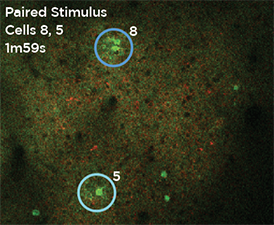
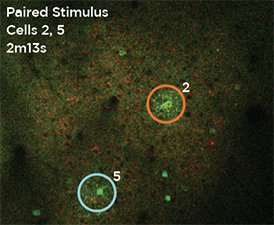
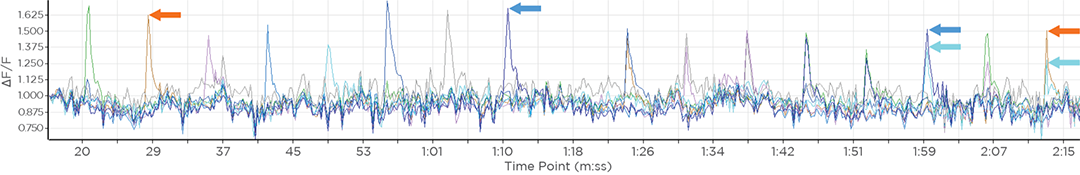
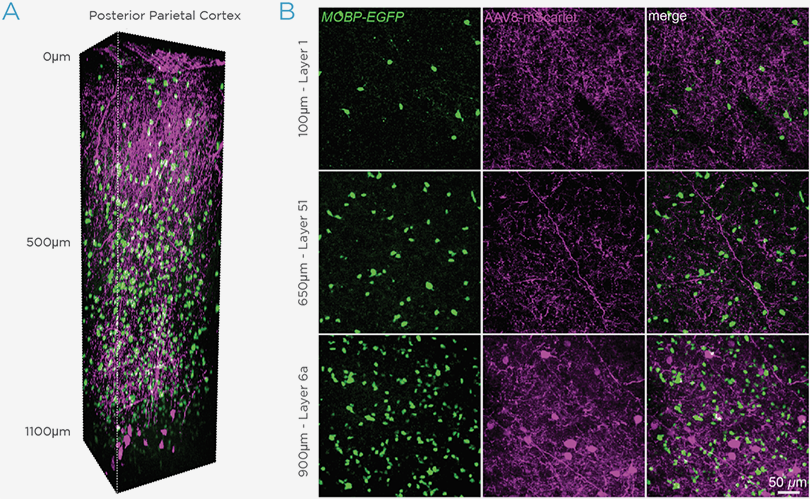
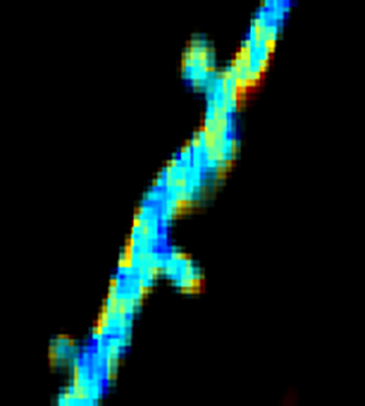
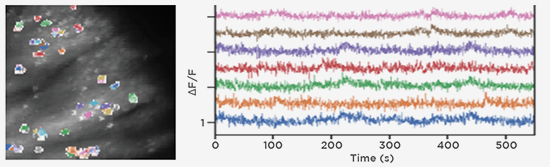
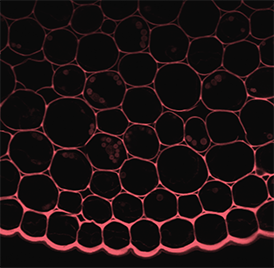
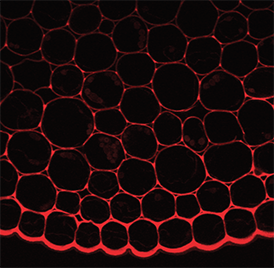
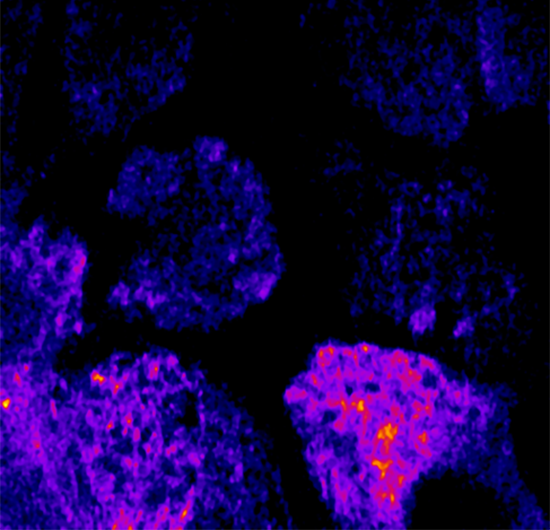
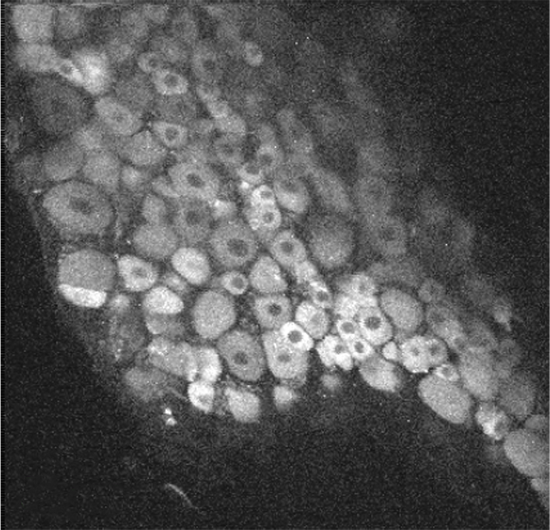
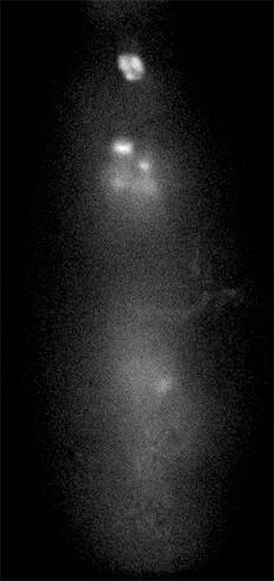
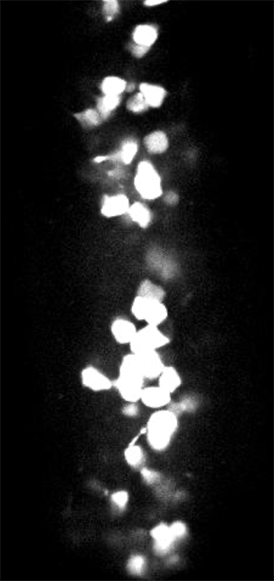
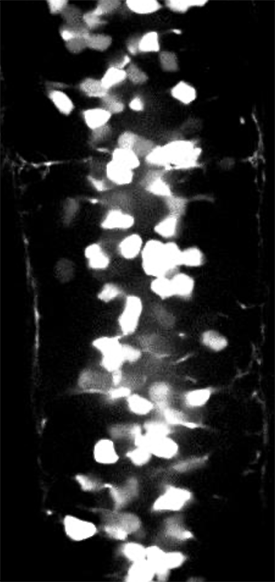
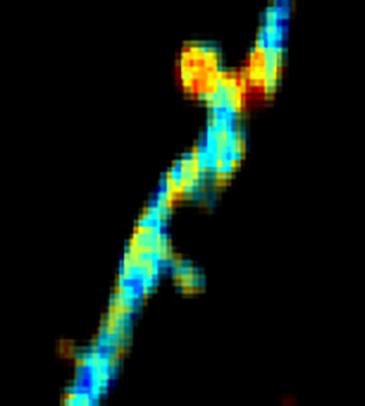GCaMP imaging of visual responses in adult Drosophila. Frye lab, UCLA Keleş and Frye, 2017. “Object-Detecting Neurons in Drosophila.” Curr Biol. 2017 Jan 30. pii: S0960-9822(17)30012-X. https://www.ncbi.nlm.nih.gov/pubmed/28190726
Imaging of electrical activity in an isolated guinea pig heart preparation. Department of Circulation and Medical Imaging, Norwegian University of Science and Technology
Weinberger et al., 2016. “Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells.” Sci Transl Med. 2016 Nov 2;8(363):363ra148.
https://www.ncbi.nlm.nih.gov/pubmed/27807283
2-photon stimulation of Chrimson in mouse cortical slices using Phasor 2-Photon, imaging with VIVO Multiphoton on a Sutter Movable Objective Microscope. Adesnik Lab, UC Berkeley
Merel et al., 2016. “Bayseian method for event analysis of intracellular currents.” J Neurosci Methods. 2016 Aug 30;269:21-32.
https://www.ncbi.nlm.nih.gov/pubmed/27208694
In vivo imaging in mouse brain through an implanted GRIN lens. Murray Lab, Louisiana Tech University
Voziyanov et al., 2016. “TRIO Platform: A Novel Low Profile In vivo Imaging Support and Restraint System for Mice.” Front Neurosci. 2016 Apr 25;10:169.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4842766/
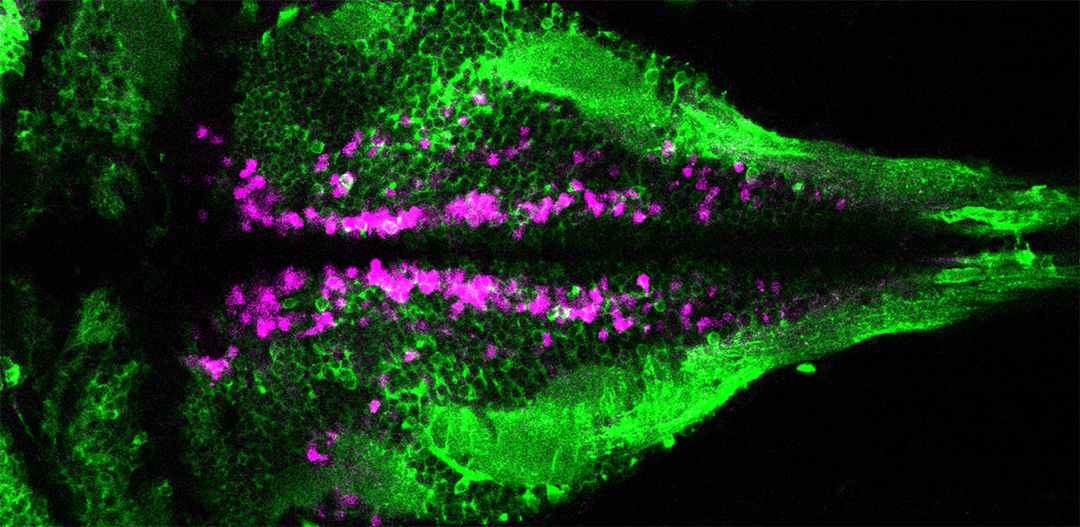
GCaMP imaging in spinal motor neurons of awake, behaving zebrafish. Wyart lab, Institut du Cerveau et de la Moelle Epinière (ICM)
Böhm et al., 2016. “CSF-contacting neurons regulate locomotion by relaying mechanical stimuli to spinal circuits.” Nat. Commun. 2015 Mar 7;7:10866.
http://www.nature.com/articles/ncomms10866
Calcium imaging in mouse brainstem slices in conjunction with electrophysiology and GABA uncaging using 488 laser line. Kandler lab, University of Pittsburgh
Weisz et al., 2016. “Excitation by Axon Terminal GABA Spillover in a Sound Localization Circuit.” The Journal of Neuroscience, 20 January 2016, 36(3):911-925.
http://www.jneurosci.org/content/36/3/911.long
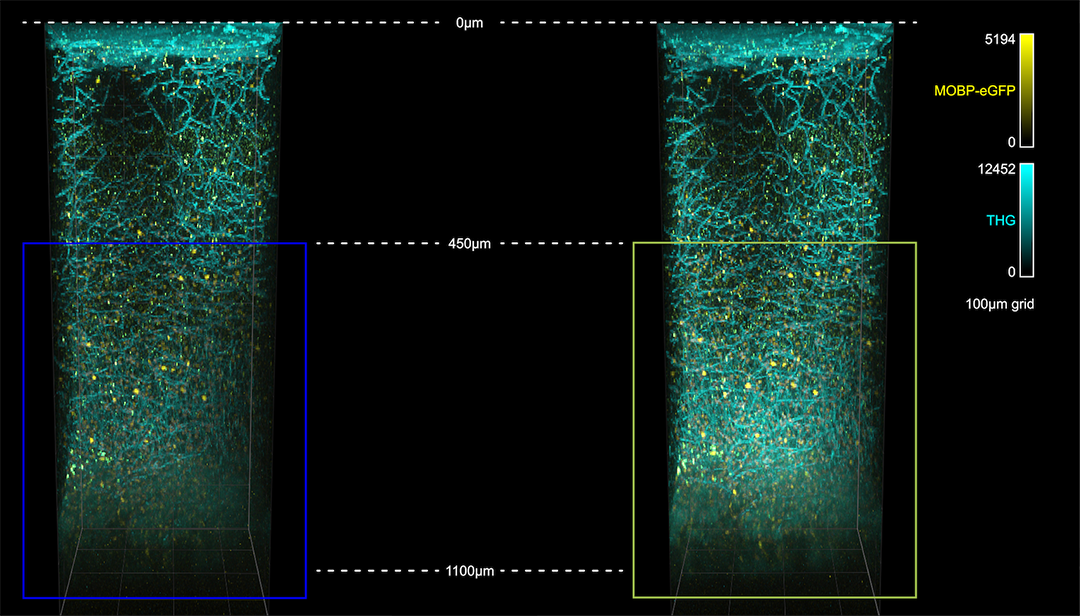
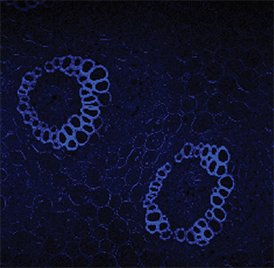
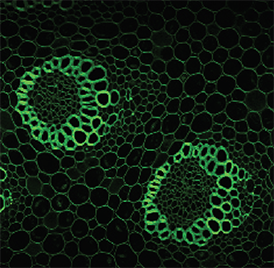
Imaging of cleared mouse spinal cord tissue. Steward Lab, UC Irvine
Willenberg and Steward, 2015. “Nonspecific labeling limits the utility of Cre-Lox bred CST-YFP mice for studies of corticospinal tract regeneration.” J Comp Neurol. 2015 Dec 15;523(18):2665-82. doi: 10.1002/cne.23809.
https://www.ncbi.nlm.nih.gov/pubmed/25976033
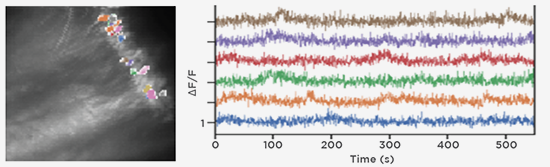
GCaMP imaging of visual responses in adult Drosophila. Frye Lab, UCLA
Aptekar et al., 2015. “Neurons forming optic glomeruli compute figure-ground discriminations in Drosophila.” The Journal of Neuroscience, 13 May 2015, 35(19): 7587-7599.
http://www.jneurosci.org/content/35/19/7587.long
GCaMP imaging in the optic lobe of adult Drosophila during visual stimulation. Frye lab, UCLA
Wasserman et al., 2015. “Olfactory Neuromodulation of Motion Vision Circuitry in Drosophila.” Current Biology , Volume 25 , Issue 4 , 467 – 472.
http://dx.doi.org/10.1016/j.cub.2014.12.012
Morphological imaging of labeled neurons in the auditory pathway in mouse brainstem slices. Kandler lab, University of Pittsburgh
Clause et al., 2014. “The precise temporal pattern of prehearing spontaneous activity is necessary for tonotopic map refinement.” Neuron , Volume 82 , Issue 4 , 822 – 835.
http://dx.doi.org/10.1016/j.neuron.2014.04.001
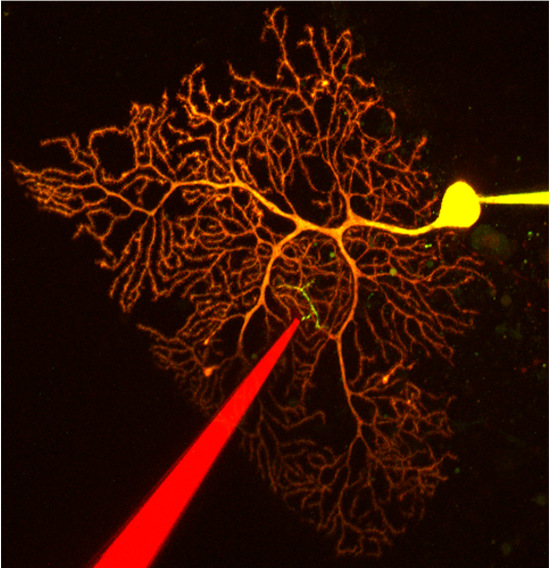
2P morphological imaging in rat brain slice combined with 1P ChR excitation and electrophysiology. Otis lab, UCLA
Mathews et al., 2012. “Effects of climbing fiber driven inhibition on Purkinje neuron spiking.” The Journal of Neuroscience, 12 December 2012, 32(50): 17988-17997.
http://www.jneurosci.org/content/32/50/17988.long